|
|
|
|
Poroelastic measurements resulting in complete data sets for granular and other anisotropic porous media |
I am now in position to develop the analogy between the isotropic and anisotropic
Gassmann (1951) equations for the case of homogeneous grains. In particular, the equation
for the suspension modulus in (2) does not change at all. In contrast,
the equation for the effective undrained bulk modulus ![]() ,
as shown in both (1) and (3),
changes only in that the relationship is now between the Reuss averages
,
as shown in both (1) and (3),
changes only in that the relationship is now between the Reuss averages
![]() and
and ![]() of these quantities. This result is completely analogous to (3),
and so will not be shown here.
of these quantities. This result is completely analogous to (3),
and so will not be shown here.
Since the remainder of the argument is virtually identical to the isotropic case, I therefore obtain:
1.2
| Stiffness | Sulfur (S) | Rochelle Salt | Benzophenone | |
| 24.0 | 25.5 | 107.0 | 215.0 | |
| 20.5 | 38.1 | 100.0 | 199.0 | |
| 48.3 | 37.1 | 71.0 | 267.0 | |
| 13.3 | 14.1 | 55.0 | 46.0 | |
| 17.1 | 11.6 | 16.9 | 22.0 | |
| 15.9 | 14.6 | 32.1 | 107.0 |
1.2
| Bulk Modulus | Sulfur (S) | Rochelle Salt | Benzophenone | |
| 20.6 | 20.1 | 54.0 | 114.6 | |
| 17.6 | 19.3 | 49.2 | 111.3 | |
| 15.2 | 12.5 | 55.8 | 87.9 | |
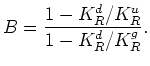 |
10.1 | 30.6 | 107.5 | 113.6 |
| 15.8 | 23.3 | 29.6 | 147.7 |
Clearly this formula does not yet provide the individual compliance matrix elements ![]() directly.
Nevertheless, Equation (49) was the hardest step in the overall procedure.
The rest of the steps follow easily once I have this rigorous result available to use.
directly.
Nevertheless, Equation (49) was the hardest step in the overall procedure.
The rest of the steps follow easily once I have this rigorous result available to use.
To finish the analysis, I make use of the newly computed value of ![]() , and substitute this
number into the formula for
, and substitute this
number into the formula for ![]() , which in this case is:
, which in this case is:
|
|
|
|
Poroelastic measurements resulting in complete data sets for granular and other anisotropic porous media |