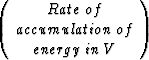 =
=
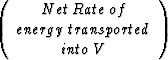 +
+
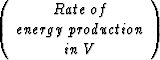
The simplified first assumption of isothermal fluid flow for hydrate structures will not be sufficient for a realistic model of the complex gas-water-hydrate system. The decomposition and growth of hydrate involves complicated thermal effects which have to be included into the fluid-flow simulation. Once the isothermal setup has been improved and is working properly, this can be achieved by extending the system with one more equation. The thermodynamics will be included based on the conservation of energy:
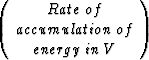 =
=
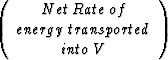 +
+
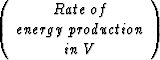
Following this equation of energy balance, the additional fluid flow equation including thermodynamics can be written as:
![]()
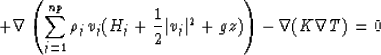 |
(31) |
The number of phases np will be given by gas, water and hydrate. The internal energy of each phase j is denoted by Uj, and the enthalpy is given by Hj. This equation will have to be added to the previous three flow equations in order to include the temperature effects on the gas-water-hydrate structure.